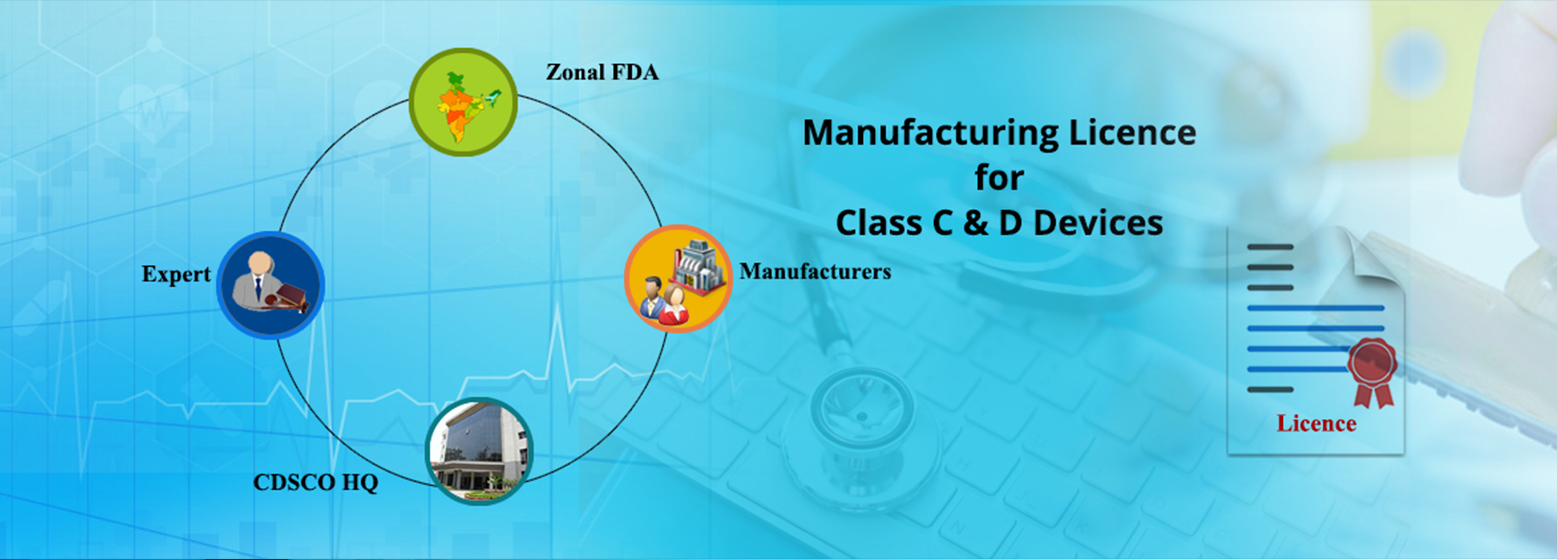
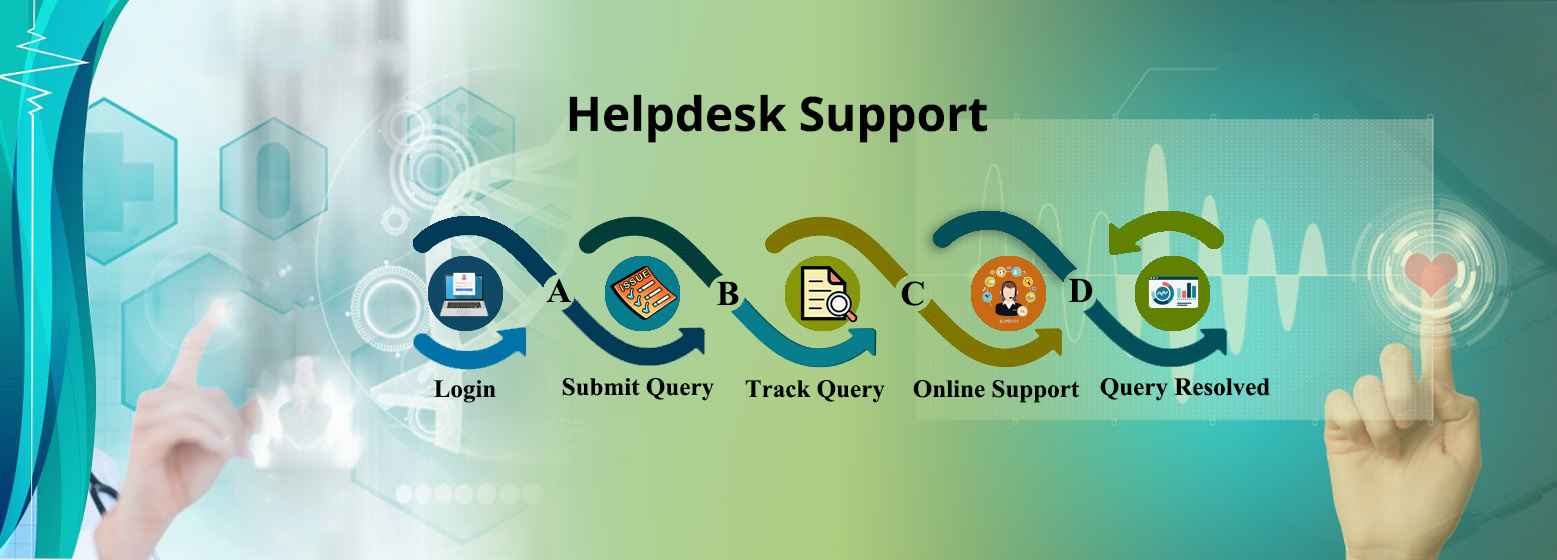
Application for Brand addition of approved devices shall be submitted separately under the provision made in the Post Approval Changes w.e.f 25.10.2024
Application for Brand addition of approved devices shall be submitted separately under the provision made in the Post Approval Changes w.e.f 25.10.2024
Note : Download User Manual For submitting Periodic Safety Update Report
About Us
The Central Drugs Standard Control Organisation(CDSCO) under Directorate General of Health Services,Ministry of Health & Family Welfare,Government of India is the National Regulatory Authority (NRA) of India. Its headquarter is located at FDA Bhawan, Kotla Road, New Delhi 110002 and also has six zonal offices,four sub zonal offices,thirteen Port offices and seven laboratories spread across the country.
Under the Drugs and Cosmetics Act, CDSCO is responsible for approval of New Drugs, Conduct of Clinical Trials, laying down the standards for Drugs, control over the quality of imported Drugs in the country and coordination of the activities of State Drug Control Organizations by providing expert advice with a view of bring about the uniformity in the enforcement of the Drugs and Cosmetics Act.

Features
Online Application Submission
Tracking of Applications
Workflow Enabled System
Timely Alerts & Notifications
Analytical & Statistical Platforms
Consumer Forms For Medical Devices
Portal Statistics